
Universitas Gadjah Mada (UGM) through the existence of the UGM Science Techno Park continues to synergize with government and industry institutions in developing various innovations that are beneficial to the vast masses. One of them was carried out in support of the government’s efforts to handle and mitigate the Covid-19 pandemic in Indonesia, namely by developing innovations in the field of medical devices in the form of domestically made breathing medical devices. The product is named Ventilator Indonesia (Venindo) code V-01 and R-03 which has successfully obtained a distribution permit number. The development of this innovation involves several ministries and strategic partners, namely the Ministry of Industry, Ministry of Health, Ministry of Research and Technology/National Research Institution, PT Swayasa Prakarsa, PT Yogya Presisi Tehnikatama Industri, PT Stechoq Robotika Indonesia, CV Rajawali 3D, and Dr. RSUP. Sardjito.
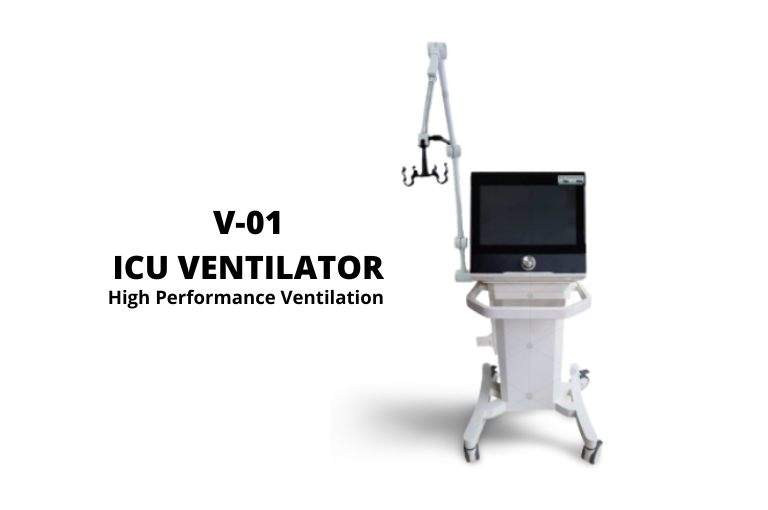
UGM together with strategic partners have succeeded in developing a ventilator with the code V-01 which is a breathing medical devices for the ICU and is categorized as high-performance ventilation. Venindo V-01 uses the internet of things and is connected to wifi to monitor air flow, oxygen, and pressure. Venindo V-01 with a 15.6” touchscreen as a monitor, has visual and audible alarms to continuously monitor tidal volume, pressure, and gas. This tool has been declared to have passed the function test by the Health Facility Supervisory Agency and has obtained a distribution permit number. The VENINDO V-01 ICU Ventilator brand has obtained a distribution permit for domestic medical devices with the RI Ministry of Health number AKD 20403220252 issued on April 20,2022 and valid until April 19, 2027. Registered under the name of PT. Swayasa Prakarsa. PT Swayasa Prakarsa is one of in-wall tennant in UGM Science Techno Park.
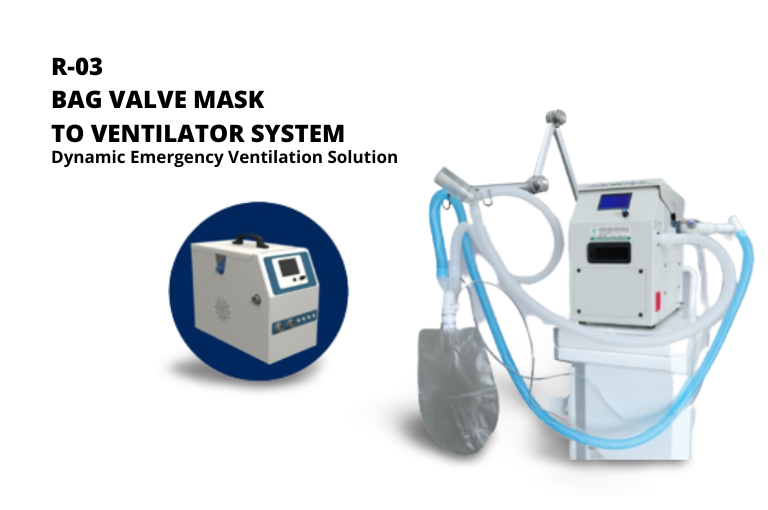
The next ventilator product which was also successfully developed by UGM together with industrial partners and has obtained a distribution permit number is Ventilator Indonesia (Venindo R-03). The ventilator with code R-03 is an emergency and transport ventilator concept based on using a bag valve mask. This ventilator functions as an invasive ventilator (with ETT intubation) and non-invasive ventilator (NIV-with a mask face). The development and production of this ventilator is expected to meet the need for safe ventilators and can be produced quickly when there is an urgent need due to the COVID-19 pandemic. This device has been declared to have passed the function test by the Health Facility Supervisory Agency and has obtained a distribution permit number. The VENINDO R-03 Modern Resuscitator Emergency Ventilator brand has obtained a distribution permit for domestic medical devices with the number KEMENKES RI AKD 20403122542 on behalf of PT. Swayasa Prakarsa. The permit was issued on December 2, 2021 and is valid until December 1, 2022.
Let’s go to our social media at
IG : @ugm.stp
Twitter : @UGMSTP
Linkedin : UGM STP




